Hi, I’m Philipp Fisch
I bioengineer human tissues inspired by developmental and extracellular matrix programs that give native tissues their structure and function.
Contact me: philippfisch91@gmail.com
Vision
The vision guiding my research is to one day regenerate human tissues by following the same developmental programs that build them in the first place. By integrating developmental and matrix biology with bioengineering, I aim to recreate tissues with native structure and function, enabling grafts that are indistinguishable from their natural counterparts. This approach shifts tissue engineering from assembling components to reimplementing the biological processes that drive tissue formation itself.
My Research
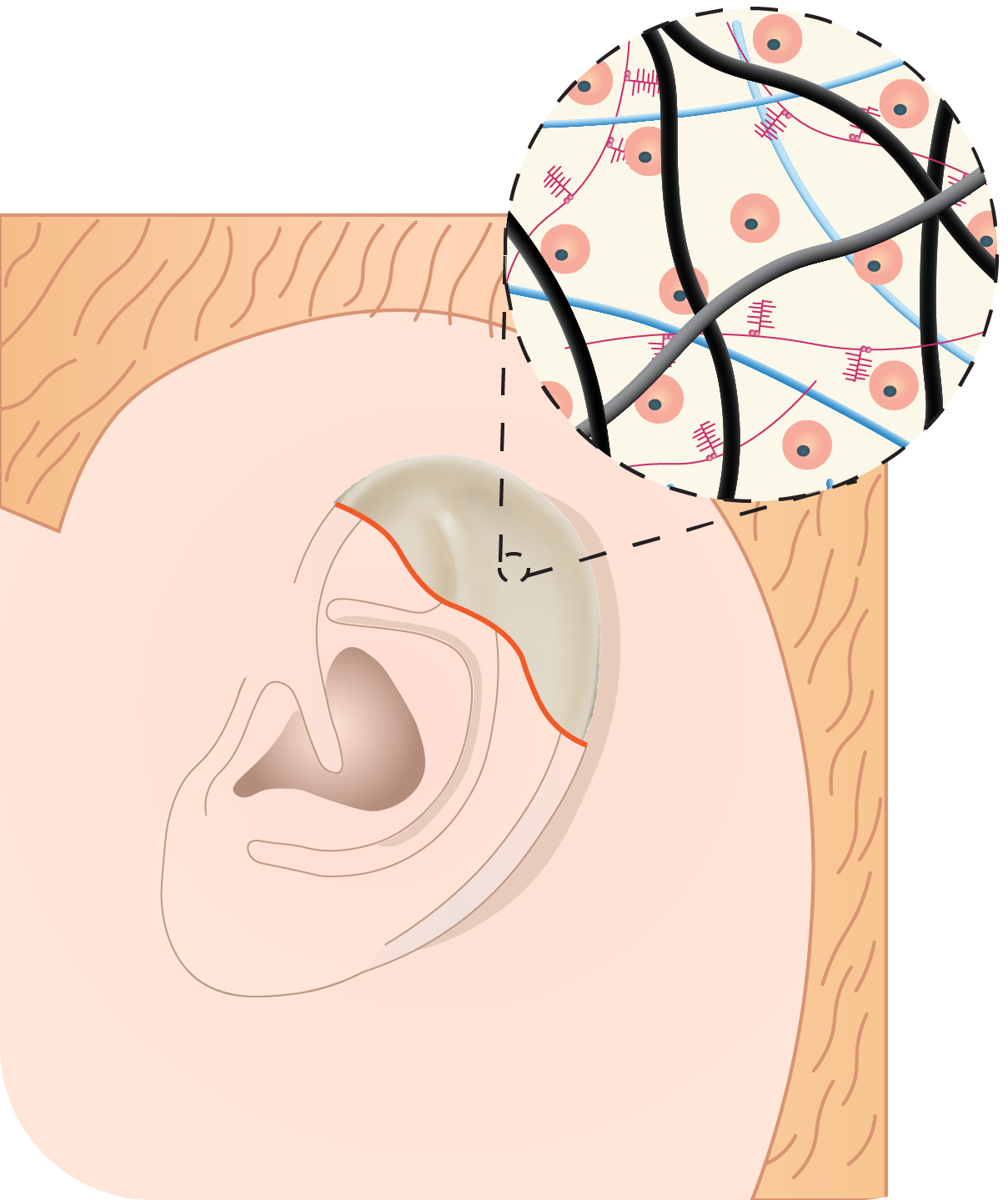
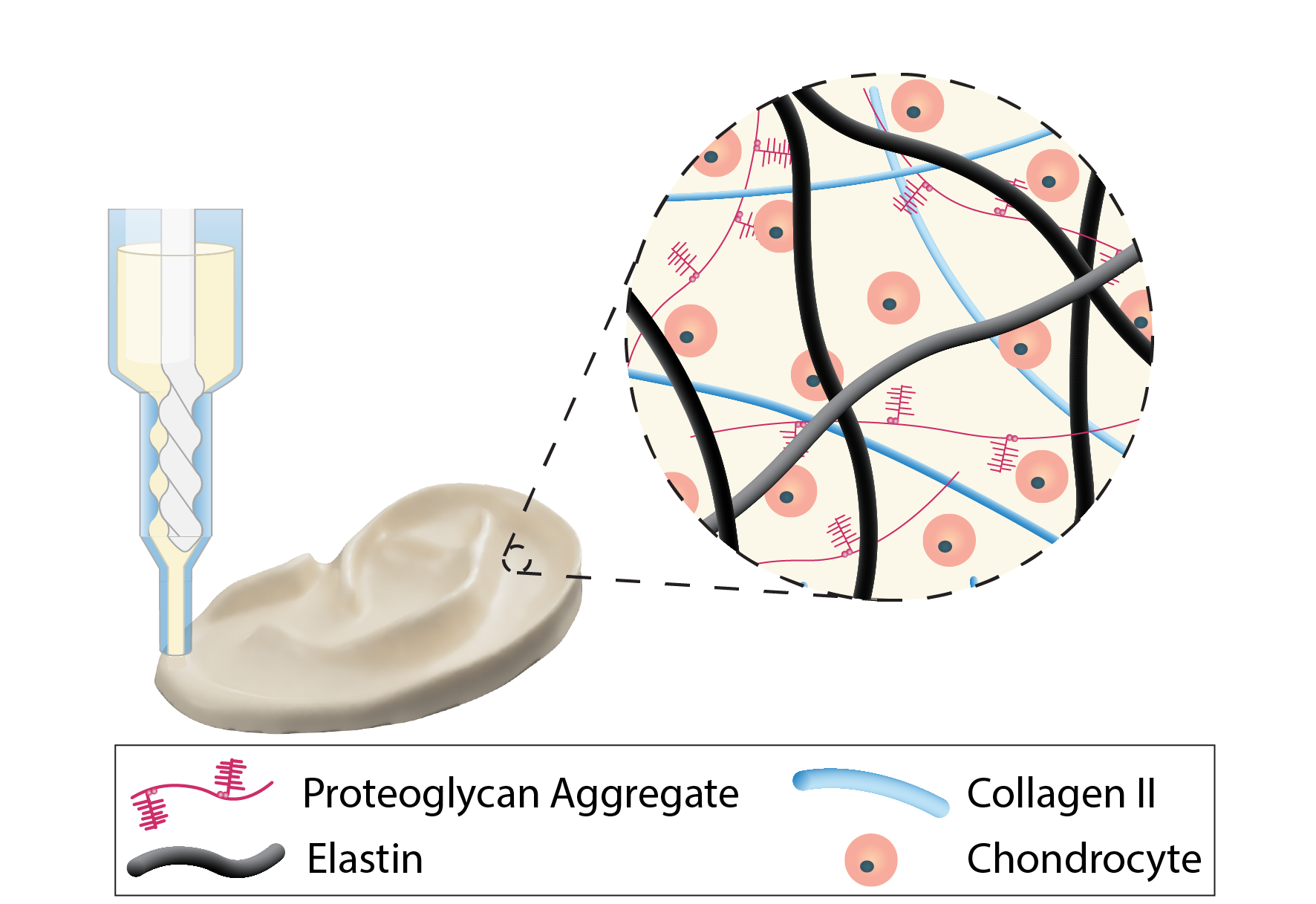
Tissue Engineered Auricular (Ear) Cartilage for the Treatment of Microtia
Microtia, the congenital malformation of the external ear, affects approximately 1 to 4 children per 10,000 births and can profoundly impact the psychosocial development during childhood. The current clinical gold standard, autologous costal cartilage reconstruction, requires the harvesting of rib cartilage and manually sculpting of an ear framework, making the procedure invasive, technically demanding, and highly dependent on surgical expertise. These limitations have motivated the past decade of my research into tissue engineered auricular grafts that could eliminate rib harvest and enable patient-specific reconstruction from 3D imaging data. Despite these efforts, incomplete tissue maturation and insufficient mechanical stability still limit their clinical translation. My research addresses this bottleneck by applying the developmental and extraceullar matrix programs that give rise to elastic cartilage to engineer auricular grafts that replicate living, human ears.
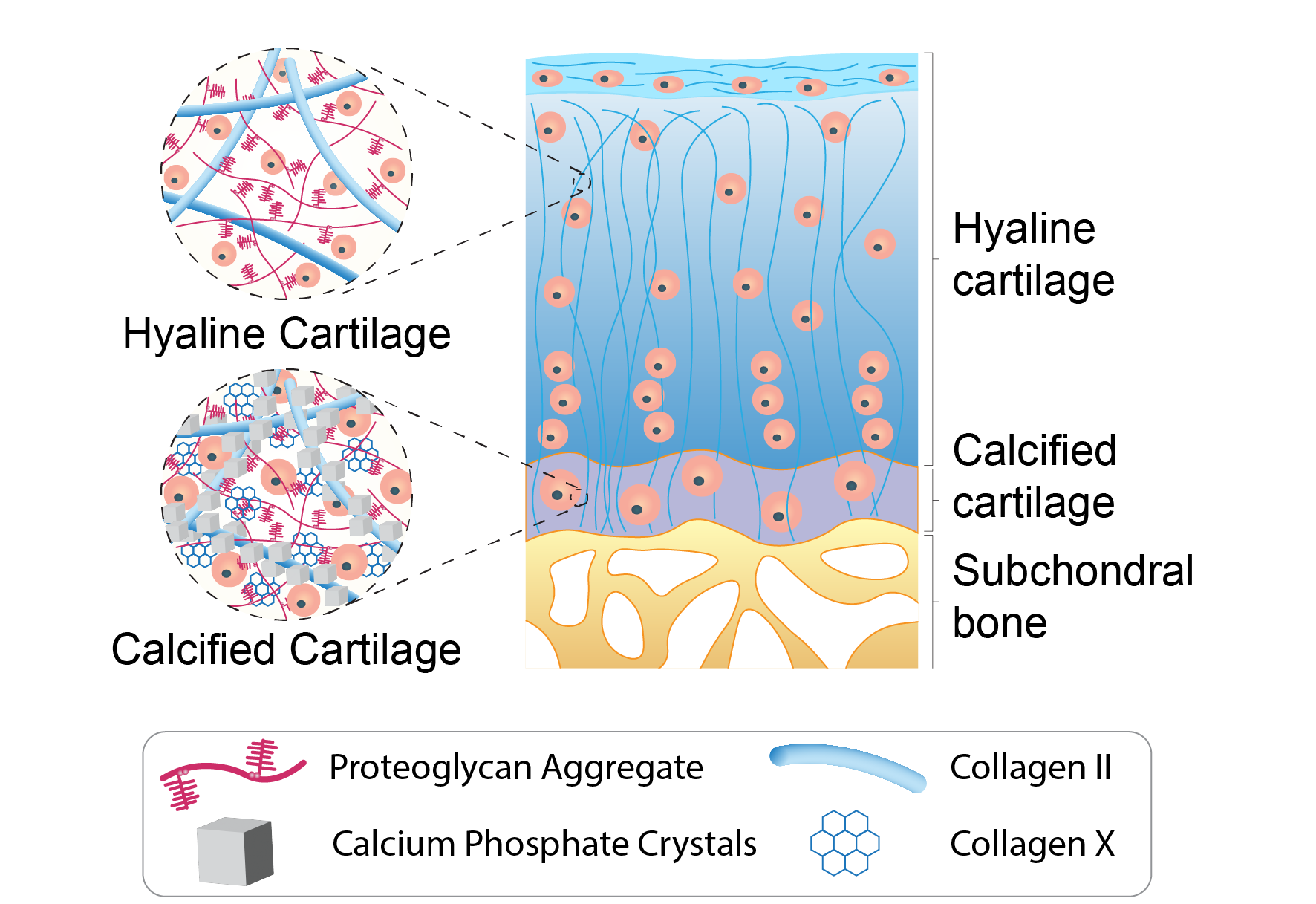
Generation of osteochondral grafts with native-tissue like properties
Osteoarthritis is a leading cause of pain and disability worldwide and arises from the progressive degeneration of the osteochondral unit, including hyaline and calcified cartilage, as well as subchondral bone. Current surgical treatments focus on symptom relief or partial repair and fail to restore the native structure and long-term function of the joint. Tissue engineered osteochondral grafts offer the potential to replace damaged tissue with living constructs that replicate the layered architecture and mechanical gradients of native joints. Despite significant advances, reproducing stable hyaline cartilage and its integration with calcified cartilage and bone remains a major challenge. My research addresses this by engineering developmentally inspired, multi-layered osteochondral grafts that enable functional tissue replacement to provide a future where we will be able to stay mobile through our lifespans.
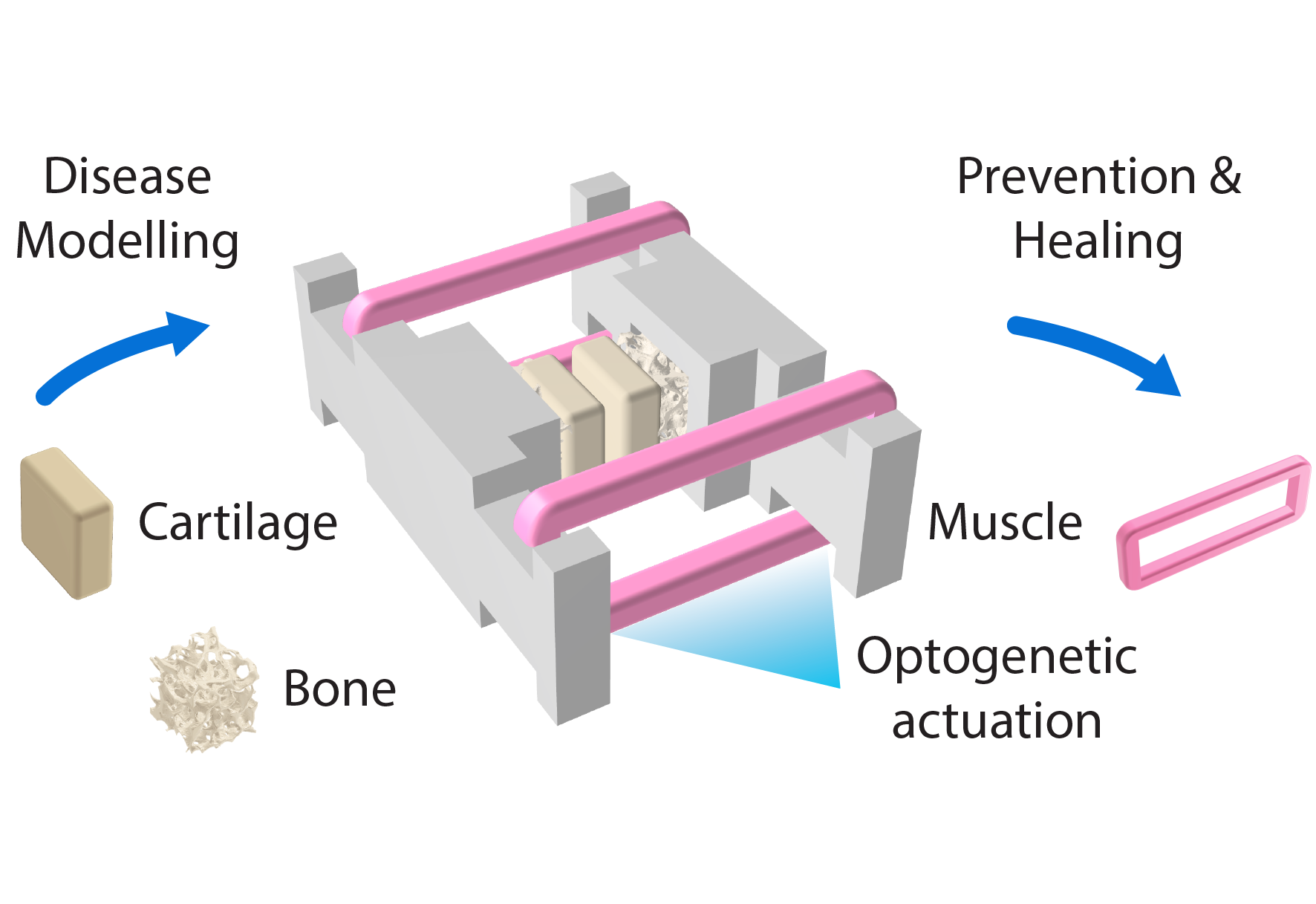
Joint-on-a-chip model for patient-specific in vitro models
Disease-modifying treatments for osteoarthritis remain elusive, in part because existing animal and in vitro models fail to capture the complex and dynamic interactions between cartilage, bone, and muscle under inflammation and mechanical loading that define human joint disease. Within the Zürich Joint initiative at ETH Zurich, I contribute to the development of a human microphysiological joint model that integrates bioengineered cartilage, bone, and optogenetically actuated muscle into a unified functional system. By reproducing physiological joint loading and inflammatory signaling in a fully human platform, this work aims to enable mechanistic studies of osteoarthritis across cellular, molecular, and mechanical scales. The long-term goal is to establish predictive, patient-specific in vitro joint models that support therapy discovery, reduce reliance on animal experimentation, and open new avenues toward disease-modifying treatments for joint degeneration. This work is conducted as part of the Zürich Joint initiative at ETH Zurich.
Publications
Tissue engineered elastic cartilage-mimetic auricular grafts for microtia reconstruction
P Fisch*, S Kessler, S Ponta, A Puigalli-Jou, G Lyu, K Flégeau, A Martyts, F Roth, D Fercher, F Rijli, D Simmen, E N Olivares, T Linder, M Zenobi-Wong
bioRxiv, 2025 (manuscript under review), https://doi.org/10.1101/2025.10.27.684810A Water-Soluble PVA Macrothiol Enables Two-Photon Microfabrication of Cell-Interactive Hydrogel Structures at 400 mm s-1,
W Qiu, M Bernero, M E Ye, X Yang, P Fisch, R Müller, X-H Qin*
Advanced Materials, 2026, https://doi.org/10.1002/adma.202510834Adipose-mesenchymal stem cells enhance the formation of auricular cartilage in vitro and in vivo
D Zielinska, K Micka-Michalak, H Ademi, P Fisch, R Boeni, T Linder, U Moehrlen, T Biedermann, A Klar*
Stem Cells Translational Medicine, 2025, https://doi.org/10.1093/stcltm/szae098Synthetic biodegradable microporous hydrogels for in vitro 3D culture of functional human bone cell networks
D Zauchner, M Z Müller, M Horrer, L Bissig, F Zhao, P Fisch, S S Lee, M Zenobi-Wong, R Müller, X-H Qin*
Nature Communications, 2024, https://doi.org/10.3929/ethz-b-000638979Evaluation of Bioprinted Autologous Cartilage Grafts in an Immunocompetent Rabbit Model
D Gvaramia†, P Fisch†, K Flégeau, L Huber, J Kern, Y, Jakob, D Hirsch, M Zenobi-Wong, N Rotter*
Advanced Therpeutics, 2024, https://doi.org/10.1002/adtp.202300441FLight biofabrication supports maturation of articular cartilage with anisotropic properties
A Puiggalí‐Jou, R Rizzo, A Bonato, P Fisch, S Ponta, D Weber, M Zenobi‐Wong*
Advanced Healthcare Materials, 2024, https://doi.org/10.1002/adhm.202302179Biofabrication of heterogeneous, multi‐layered, and human‐scale tissue transplants using eluting mold casting
E Tosoratti, D Rütsche, M Asadikorayem, S Ponta, P Fisch, K Flégeau, T Linder, P Guillon, M Zenobi‐Wong*
Advanced Functional Materials, 2024, https://doi.org/10.1002/adfm.202305651Suitability of ex vivo-expanded microtic perichondrocytes for auricular reconstruction
Y Jakob, J Kern, D Gvaramia, P Fisch, R Magritz, S Reutter, N Rotter*
Cells, 2024, https://doi.org/10.3390/cells13020141Combining bio-engineered human skin with bioprinted cartilage for ear reconstruction
D Zielinska†, P Fisch†, M Zenobi-Wong, T. Biedermann, A Klar*
Science Advances, 2023, https://doi.org/10.1126/sciadv.adh1890Engineering inflammation‐resistant cartilage: bridging gene therapy and tissue engineering
A Bonato, P Fisch, S Ponta, D Fercher, M Manninen, D Weber, K Eklund, G Barreto, M Zenobi-Wong*
Advanced Healthcare Materials, 2023, 2202271, https://doi.org/10.1002/adhm.2022022713D-Printed reinforcement scaffolds with targeted biodegradation properties for the tissue engineering of articular cartilage
E Tosoratti†, P Fisch†, S Taylor, M Zenobi-Wong*
Advanced Healthcare Materials, 2021, https://doi.org/10.1002/adhm.202101094Bioprinting of cartilaginous auricular constructs utilizing an enzymatically crosslinkable bioink
P Fisch, N Broguiere, S Finkielsztein, T Linder, M Zenobi-Wong*
Advanced Functional Mater., 2021, https://doi.org/10.1002/adfm.202008261Improved accuracy and precision of bioprinting through progressive cavity pump-controlled extrusion
P Fisch, M Holub, M Zenobi-Wong*
Biofabrication, 2020, https://doi.org/10.1088/1758-5090/abc39bCell-laden agarose-collagen composite hydrogels for mechanotransduction studies
E Cambria, S Brunner, S Heusser, P Fisch, W Hitzl, S Ferguson, K Wuertz-Kozak*
Frontiers in Bioengineering and Biotechnology, 2020, https://doi.org/10.3389/fbioe.2020.00346Development and thorough characterization of the processing steps of an ink for 3D printing for bone tissue engineering
M Mueller, P Fisch, M Molnar, S Eggert, M Binelli, M Zenobi-Wong*
Materials Science and Engineering: C, 2020, https://doi.org/10.1016/j.msec.2019.110510A comparative study of cartilage engineered constructs in immunocompromised, humanized and immunocompetent mice
E Cavalli†, P Fisch†, F Formica, R Gareus, T Linder, L Applegate, M Zenobi-Wong*
Journal of Immunology and Regenerative Medicine 2018, https://doi.org/10.1016/j.regen.2018.09.001Cartilage tissue formation through assembly of microgels containing mesenchymal stem cells
F Li, V Truong, P Fisch, C Levinson, V Glattauer, M Zenobi-Wong, H Thissen, J Forsythe, J Frith,
Acta Biomaterialia, 2018, https://doi.org/10.1016/j.actbio.2018.07.015Guidelines for standardization of bioprinting: a systematic study of process parameters and their effect on bioprinted structures
M Kesti, P Fisch, M Pensalfini, E Mazza, M Zenobi-Wong*
BioNanoMaterials, 2016, https://doi.org/10.1515/bnm-2016-0004
† contributed equally to this work, * corresponding author
I am a bioengineer working at the interface of biology and engineering with the goal of regenerating living tissues that restore native structure and function. I trained in mechanical and biomedical engineering at ETH Zurich, where I developed a strong foundation in mechanics, materials, and biofabrication, and completed my PhD in tissue engineering and biofabrication with Prof. Marcy Zenobi-Wong. My early work focused on developing biofabrication strategies for complex cartilaginous tissues and revealed a central limitation of the field: engineered tissues rarely reach biological maturity.
As a postdoctoral and senior researcher at ETH Zurich, I shifted my focus toward biological maturation and translation, leading multidisciplinary efforts on tissue engineered auricular and osteochondral grafts and securing competitive translational and academic funding. To expand my scientific scope, I completed a research stay at Stanford University in the laboratory of Prof. Mark Skylar-Scott, where I developed gene-driven stem cell differentiation strategies inspired by developmental programs. My work now centers on integrating developmental and matrix biology with bioengineering to establish new paradigms for functional tissue regeneration, with the long-term goal of building an independent research program under the umbrella of Developmental Bioengineering and Regeneration.
About Me
Senior Researcher, ETH Zurich, 2024-Present
Visiting Scholar, Stanford University, 2024-2025
Postdoctoral Scholar, ETH Zurich, 2022-2024
Dr. sc., ETH Zurich, 2016-2022
MSc Biomedical Engineering, ETH Zurich , 2014-2016
BSc Mechanical Engineering, ETH Zurich, 2011-2014